
If nucleus of an atom is unbalanced, that is, if number of protons and neutrons differs, atom is unstable. It accomplishes this by emitting particles such as alpha and beta. Process through which unstable nucleus of an atom loses energy by emitting particles is known as radioactivity. However, findings of subsequent trials contradicted its assertions. Atomic model of Thomson was successful in explaining atom's general neutrality. Assumption that atom's mass is equally distributed across atom is key feature of this model. As a result, watermelon model, plum pudding model, and raisin pudding model are all names for this model. The above illustration resembles a chopped watermelon with electrons representing seeds. Electrons are encased in this sphere to create stable electrostatic configuration possible. Thomson proposed that atom is shaped like a sphere with radius of around 10-10m with uniformly distributed positive charge. Cathode rays were found to be negatively charged as a result of this.

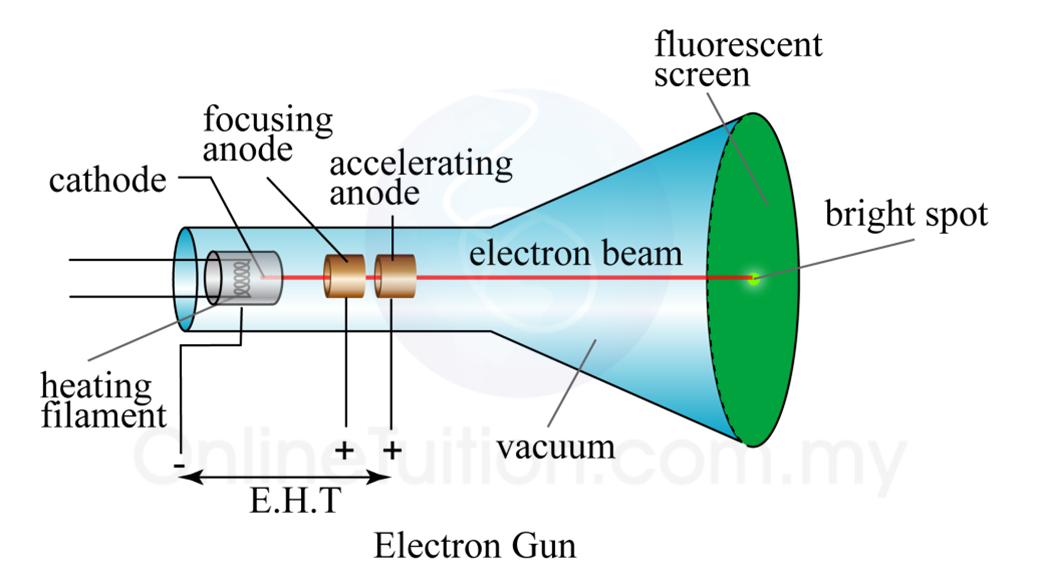
He discovered that when he used charged metal plates, cathode rays bent away from negative plate and towards positive plate. Thompson used fluorescent coated tube instead of electrometer at one end of Cathode Ray Tube, which would illuminate when cathode ray hit it. He now placed a negatively charged metal plate on one side of cathode rays to allow them to pass through anode and a positively charged metal plate on the other. He then performed a second experiment to determine whether charge carried by cathode rays was negative or positive. He observed that electrometers ceased measuring electric charge after first experiment. Two microscopic perforations in metal led to an electrometer that could measure a modest electric charge. On the other end of his cathode ray tube, he created a metal cylinder. Thompson performed the first cathode ray tube experiment to demonstrate that rays released by an electron cannon are inseparable from latent charge. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City.J.J. We also see two other tubes with Maltese-cross cathodes ( third image), and a view of a worship producing Crookes tubes in 1896 ( fourth image). The first image shows a display of original Crookes tubes at the MUMOK, the Museum of Modern Art, in Vienna. In 1903, Vanity Fair published a colorful caricature of Crookes, which shows both his splendid beard and mustache, and his tube ( second image). So Crookes’ device made quite an important contribution to what we often call the second scientific revolution, the birth of atomic physics. Roentgen had used the Crookes tube to discover X-rays. At about the same time, William Roentgen found that when the cathode rays strike the end of the tube, they produce radiation that can pass right through the tube wall. Using a Crookes tube, and deflecting the cathode rays by a magnetic field, Thomson discovered that the cathode rays were actually very tiny particles with a negative charge. Thomson at the Cavendish Lab in Cambridge who, in 1897, figured out what was happening. Researchers made them in all shapes and sizes, as much for entertainment as anything, but no one really understood what was going on inside the tube. Crookes had invented the cathode-ray tube, although at the time it was universally referred to as a “Crookes tube”. Around 1870, Crookes invented a partially-evacuated glass tube that contained two electrodes when a high voltage was applied, the remaining gases in the tube would glow, and if certain phosphorescent materials were painted on the inside of the tube, parts of the tube would glow as well. William Crookes, an English chemist, was born June 17, 1832.


 0 kommentar(er)
0 kommentar(er)
